Ensuring compliance with the EU MDR can be a complex task for medical device companies. An EU MDR checklist is essential for systematically managing regulatory requirements and streamlining your compliance process. Whether you’re a quality manager or regulatory affairs specialist, these checklists help you track documentation, assess risk management, and ensure product safety.
By using an EU MDR checklist, you maintain consistency and thorough documentation, which are crucial for avoiding costly compliance issues. Without a structured approach, you might overlook critical regulatory requirements, leading to delays and non-compliance risks. This templates allows you to efficiently navigate the regulatory landscape, ensuring that your products meet all necessary standards.
EU MDR checklist templates
Explore our comprehensive templates designed to streamline compliance with the European Union Medical Device Regulation. These templates cover essential aspects of the regulation, helping you ensure thorough documentation and adherence to standards:
EU MDR checklist
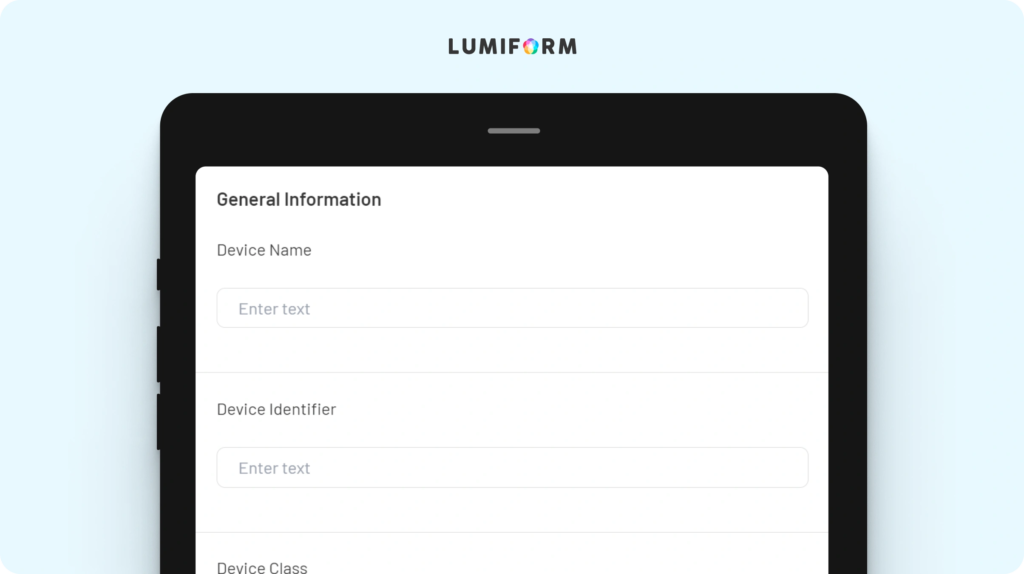
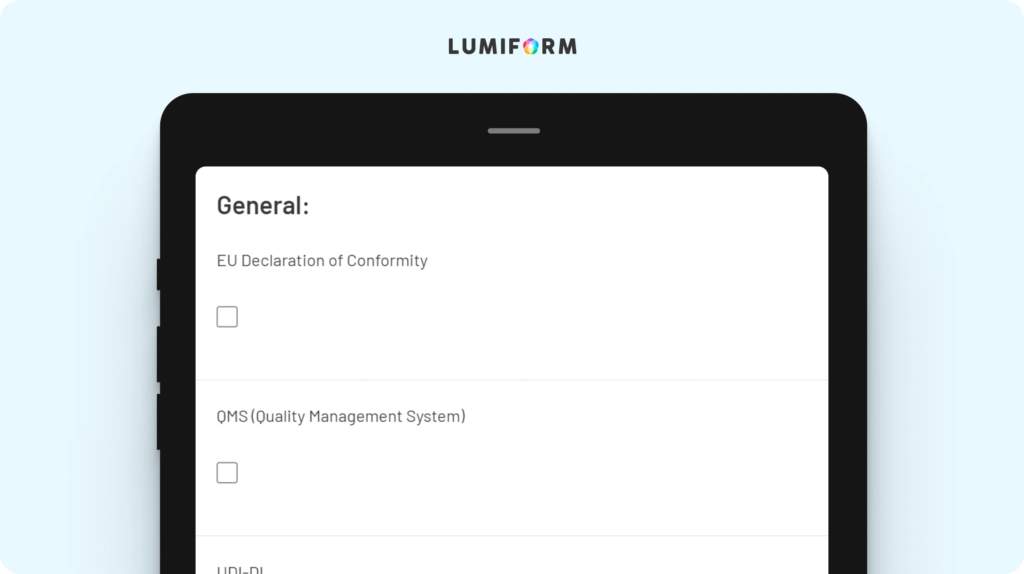
The EU MDR checklist is designed to help your team systematically manage compliance with the European Medical Device Regulation. It includes key features such as documentation tracking, risk assessment, and product safety verification. Customize it to align with your specific regulatory needs and product types, ensuring a comprehensive compliance process. This tool helps you maintain consistency and avoid potential compliance issues.EU MDR essential requirements checklist
This checklist focuses on the essential requirements of the EU MDR, providing a structured approach to ensure your products meet all necessary standards. Key features include sections for safety, performance, and labeling requirements. Customize it to reflect the specific requirements of your devices, ensuring thorough compliance and reducing the risk of regulatory setbacks.EU MDR gap analysis template
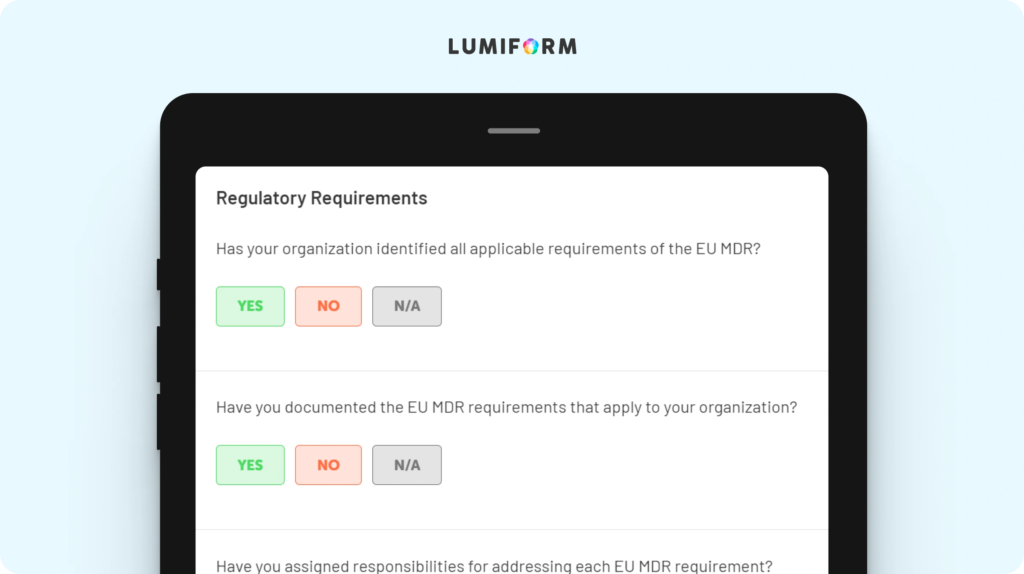
The EU MDR gap analysis template helps you identify and address gaps in your current compliance strategy. It features sections for regulatory requirements, current compliance status, and action plans. Customize it to focus on areas most relevant to your organization, ensuring a targeted approach to closing compliance gaps and enhancing regulatory readiness.EU MDR clinical evaluation report template
The EU MDR clinical evaluation report template provides a framework for documenting clinical data and evaluations. Key features include sections for clinical evidence, risk-benefit analysis, and clinical performance. Customize it to align with your specific device and clinical data, ensuring a comprehensive and compliant clinical evaluation process that supports regulatory submissions.
How to create an EU MDR checklist template in Lumiform
Creating an EU MDR checklist template in Lumiform is straightforward, ensuring your team can efficiently manage compliance tasks. Start by using digital forms on mobile to make the checklist easily accessible for your team, allowing them to conduct compliance checks anywhere, anytime.
Incorporate logic and action management to guide users through the checklist based on their inputs, ensuring all regulatory requirements are addressed without missing critical steps. Use role assignment to designate specific tasks to team members, enhancing accountability and ensuring that each part of the checklist is completed by the right people.
To make the template user-friendly, include instructions and QR codes. These features help users quickly understand the checklist requirements and navigate efficiently. By focusing on these elements, you create a template that not only improves the thoroughness of your compliance process but also ensures your team can handle regulatory tasks effectively and confidently.