ISO 17025 is an international standard for testing and calibration laboratories, and meeting the requirements means that your lab is competent, consistent, and capable of producing reliable results. An ISO 17025 checklist helps you break down the requirements into clear, actionable steps so you can have a more organized process.
From assessing quality management systems to making sure that every piece of equipment is calibrated, ISO 17025 compliance demands meticulous attention to detail. With the checklists below, you’ll find it easier to stay on track and monitor your documentation.
Top ISO 17025 checklist templates
Make compliance comprehensive and manageable with these templates:
ISO 17025 checklist
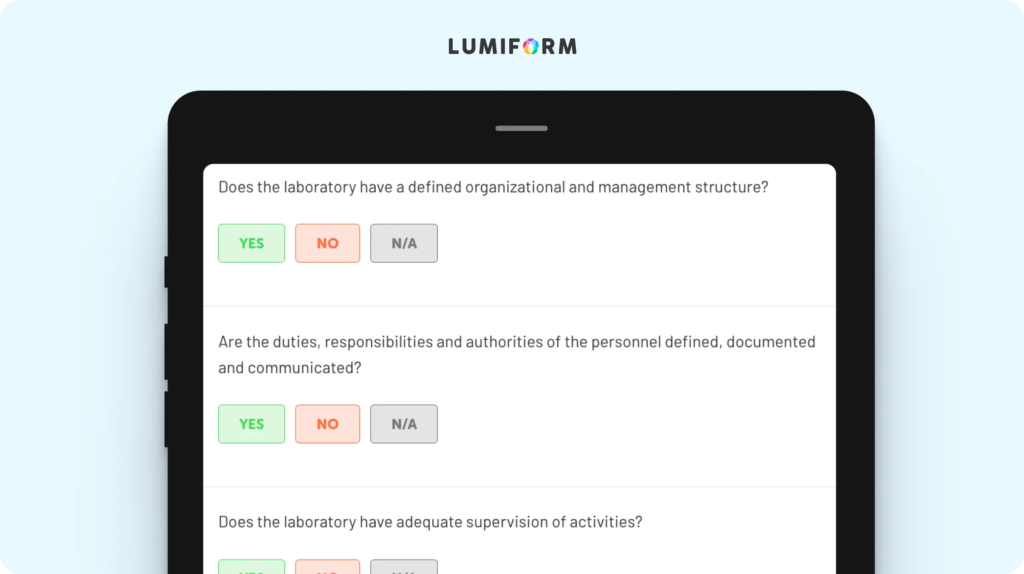
This template converts the ISO 17025 standard’s complex requirements into smaller tasks so your lab can stay more organized during audits, assessments, or day-to-day operations. It covers quality management processes, equipment calibration, and personnel competency, addressing critical elements of the standard. You can easily adapt the checklist to your lab’s unique workflows by adding custom fields for specific procedures, timelines, or documentation requirements. By tailoring it to fit your operations, this checklist becomes a powerful tool for maintaining compliance and consistency, no matter the scope of your work.ISO 17025 gap analysis checklist
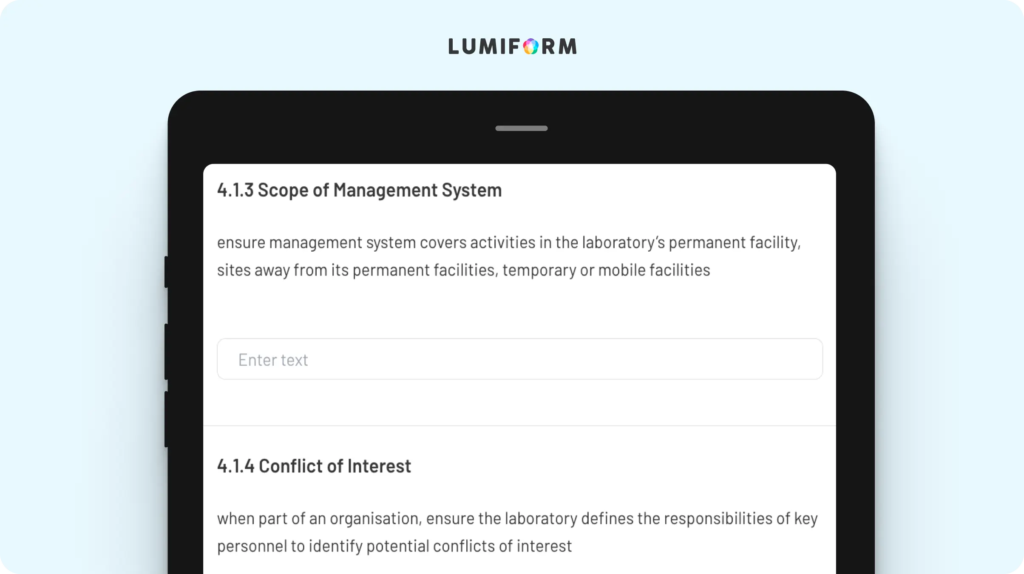
Closing compliance gaps is easier with this gap analysis checklist, which helps you identify where your lab meets—or falls short of—ISO 17025 requirements. It’s designed to guide you step-by-step through the standard, pinpointing areas that need improvement, from calibration processes to document control. By customizing the checklist to reflect your lab’s specific procedures and resources, you can prioritize action items and create a clear roadmap for achieving full compliance. With this tool, you can focus your efforts on the areas that need the most attention.ISO 17025 internal audit checklist
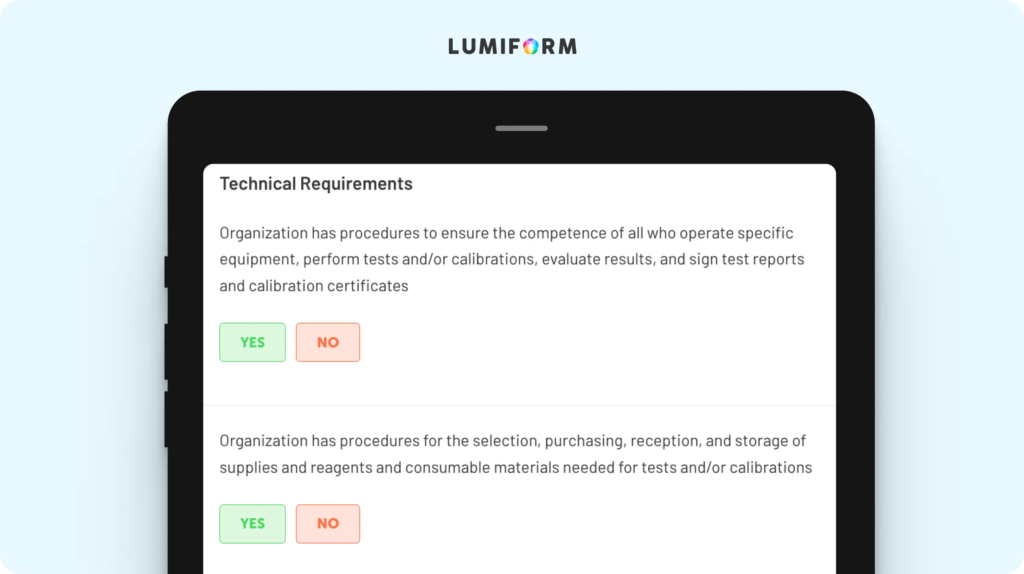
Internal audits are a key part of ISO 17025 compliance, and this template ensures that yours are thorough, efficient, and stress-free. The checklist reminds you to review your quality management system and verify equipment calibration and personnel qualifications so you can evaluate your lab’s readiness for external audits. Customizing it is simple—add or adjust sections to reflect your lab’s unique processes and audit schedules. With this template, you’ll have a structured framework for identifying non-conformities, documenting findings, and implementing corrective actions.ISO 17025 management review template
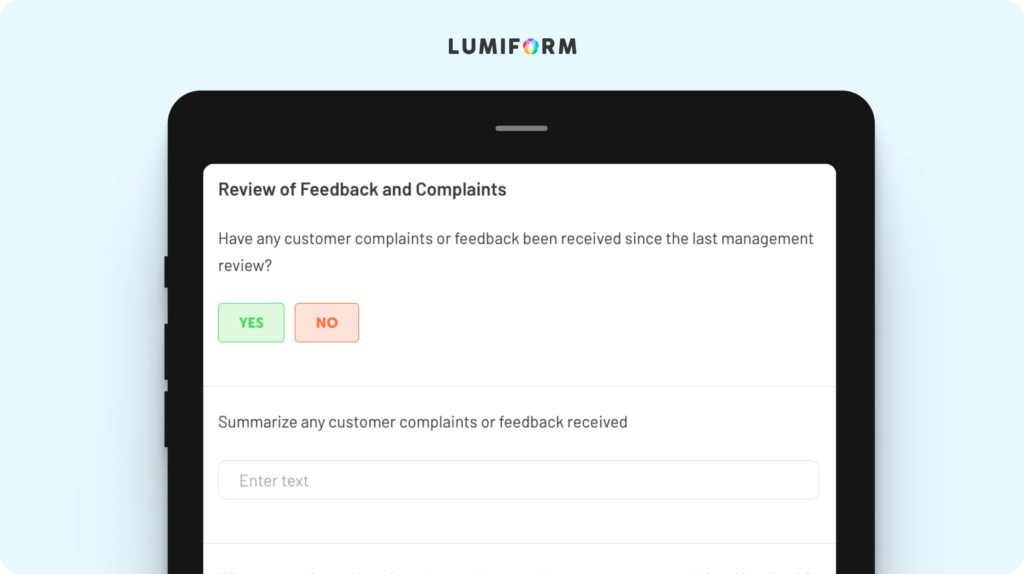
Effective management reviews are key to maintaining ISO 17025 compliance, and this template ensures that meetings are productive and aligned with the standard’s requirements. It offers a systematic format for discussing audit results, resource needs, corrective actions, and overall lab performance. You can tailor the template to include specific metrics, key performance indicators, or agenda items that reflect your lab’s priorities. By using this framework, your management team can make informed decisions that drive continuous improvement and ensure compliance with ISO 17025.ISO 17025 quality manual template
A well-crafted quality manual is fundamental to ISO 17025 compliance, serving as a detailed reference for your lab’s procedures, policies, and objectives. This template provides a comprehensive structure, from your lab’s scope of accreditation to document control and quality management processes. It’s fully customizable, so you can adapt it to reflect your lab’s unique operations, terminology, and workflows. This way, you create a practical guide for both staff and auditors, leading to consistency in your lab’s work.ISO 17025 standard operating procedures template
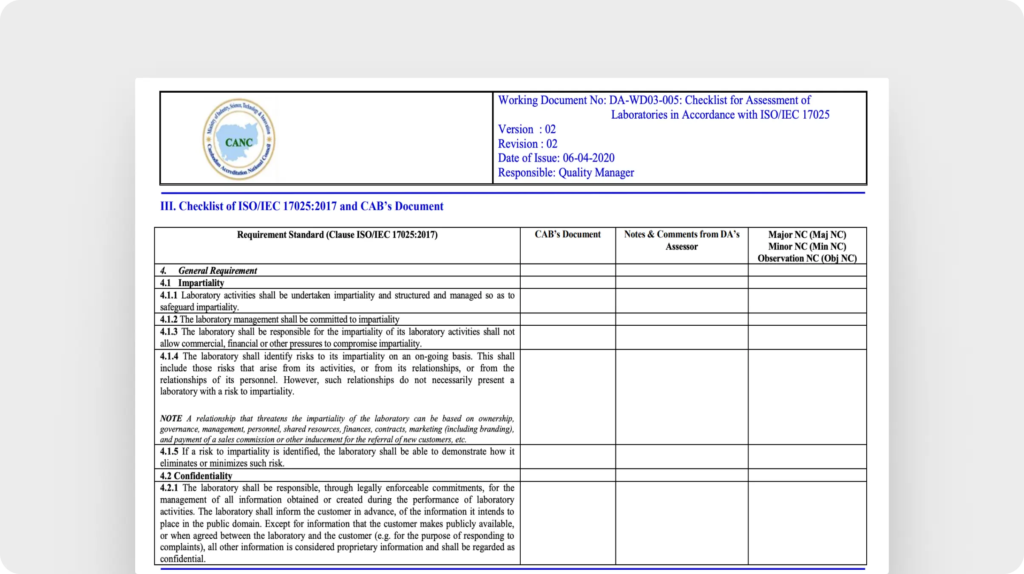
With clear and consistent standard operating procedures (SOPs), tasks in your lab can be performed with more precision and accuracy. This SOP template guides you through documenting step-by-step procedures, including sample handling, equipment calibration, and testing methods. It’s designed to help your lab standardize processes, reducing the risk of errors or non-conformities.Checklist for assessment of laboratories in accordance with ISO/IEC 17025 (CANC)
To ensure your lab meets the highest international standards, you can reference this iSO 1725 checklist from the the Cambodian Accreditation National Council’s assessment tool. It’s designed help you evaluate laboratory competence with clarity and confidence. From management requirements to technical procedures, the sections align with the standard’s core criteria, making your audit preparation easier. It acts as a step-by-step guide to identify gaps, improve documentation, and foster continuous improvement.
How to create an ISO 17025 checklist in Lumiform
Creating your own ISO 17025 template in Lumiform is simple and effective.
Start by selecting any of the templates above or setting up a new one with the AI form builder. This lets you design a customized template tailored to your lab’s processes. Add multiple input types, like text fields for calibration notes, number fields, or photo uploads to document equipment conditions.
Once your template is set up, you can also access it through Lumiform’s mobile app. This makes it easy for team members to complete forms directly in the lab or during audits. Found a problem? You can flag issues in real time and assign corrective tasks to the right person, ensuring quick action.
Afterward, use the data collected to generate detailed reports. You’ll be able to analyze trends and identify areas for improvement without digging through unnecessary paperwork.